News & Events
React4life Updates
Check the latest news and information about our company and technology. Find here news about our products, services and training. Read our articles, check our latest events and clips from the press.
React4Life is a founding member of IAMPS, Europe’s first MPS industry alliance
February 6, 2026
React4Life is one the 9 founding members of IAMPS, the world's first industry alliance for Microphysiological Systems. A strategic step to position Italy and Europe at the forefront of the transition toward more predictive, ethical, and human-relevant biomedical research.
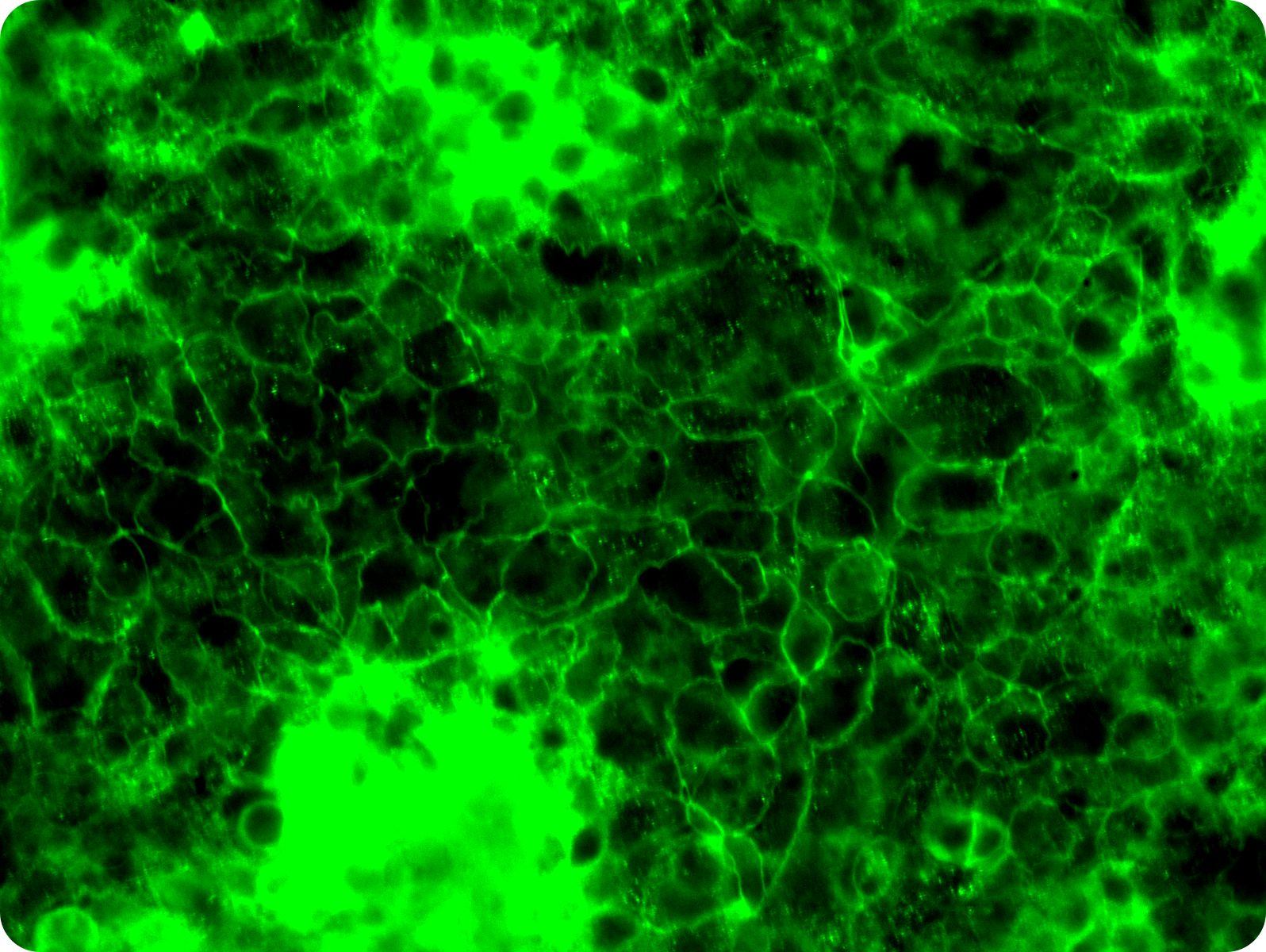
A new milestone in Gut Barrier Research: The MIVO® Double Flow Gut-on-chip
November 28, 2025
Discover how the MIVO® Gut-on-Chip brings intestinal research to the next level. By combining dual-flow physiology, tunable mucus production, and rapid barrier maturation, this advanced platform delivers human-relevant insights for drug absorption, microbiota interaction studies, and disease modeling—while reducing reliance on animal testing.
React4life and Prodeco Pharma Announce Exciting Partnership in Evidence-Based Botanical Research
October 30, 2025
React4life partners with Prodeco Pharma, combining the patented MIVO® organ‑on‑chip platform with phytotherapy expertise to accelerate evidence‑based research and development of botanical supplements, nutraceuticals and dermocosmetics.
Breaking barriers in biotech – the EIC Women Leadership Programme and React4Life’s co‑founder Silvia Scaglione
October 29, 2025
Silvia Scaglione’s interview on the European Innovation Council Women Leadership Programme is now live. Read the full story on the EIC site.
Mac4Me project kicks off with a meeting in Rotterdam and the first doctoral training session
July 11, 2025
The Mac4Me project officially took off in Rotterdam, where Erasmus University Medical Center hosted its kick-off meeting.
Bringing together international partners and doctoral researchers, this Horizon Europe MSCA initiative aims to redefine metastatic cancer research through macrophage-targeted innovation.
FDA Phases Out Animal Testing: React4life at the Forefront of Drug Innovation
May 5, 2025
The FDA’s move to phase out animal testing in monoclonal antibody development marks a major shift in regulatory science, highlighting New Approach Methodologies (NAMs). React4life is ready to lead this transition, advancing a future where drug development is faster and more predictive
React4life awarded the “Premio America Innovazione” at the Italian Parliament
April 23, 2025
React4life is proud to be honored with the America Innovation Award by the Italy–USA Foundation.
This recognition underscores our contribution to scientific innovation through MIVO® organ-on-chip technology and strengthens Italy–USA collaboration in research and industry.
Mac4Me Project: Macrophage Targets for Metastatic Treatment
February 21, 2025
Mac4Me, an innovative Horizon Europe Marie Skłodowska-Curie Actions Doctoral Network, brings together 14 international partners to advance research on metastatic cancer. Focusing on neuroblastoma, breast, and prostate cancer, Mac4Me uses cutting-edge organ-on-chip models and AI to identify immune targets, improving immunotherapy efficacy against metastatic disease.
SLAS INTERNATIONAL 2025
January 20, 2025
React4life attended the 2025 SLAS conference in San Diego.
The event connects scientists with the latest laboratory automation and screening technologies shaping the future of research.
Premio 2031: React4life Recognized for Innovation Among Italy’s Leading Pioneers
January 3, 2025
React4life is proud to be honored with the America Innovation Award by the Italy–USA Foundation.
This recognition underscores our contribution to scientific innovation through MIVO® organ-on-chip technology and strengthens Italy–USA collaboration in research and industry.