Skin absorption assays are crucial for assessing the absorption kinetics of various molecules. Absorption is a critical factor determining the bioavailability of a compound, as it needs to permeate the skin barrier to reach the bloodstream and exert its biological effects. These assays aim to demonstrate successful delivery of active ingredients, predict risks related to exposure to toxic molecules, or confirm the absence of absorption of specific substances. They are also crucial for evaluating, classifying, risk assessing, and determining the intended use of medical devices.
According to the definition of “medical device”, it is characterized by two main features: (i) therapeutic and/or diagnostic medical purpose, and (ii) main mechanism of action: non-pharmacological, neither immunological, nor metabolic. According to EU Regulation 2017/745, a new classification refers to medical devices composed of “substances or combinations of substances that are intended to be introduced into the human body through a body orifice or applied to the skin and that are absorbed by or locally dispersed into the human body”. Specifically, if such devices are applied on the skin or in the nasal or oral cavity up to the pharynx, they shall be classified as class IIa; whereas, they shall be classified as class III (highest risk) if they are systemically absorbed by the human body to achieve their intended purpose, or if they achieve their intended purpose in the stomach or lower gastrointestinal tract and are systemically absorbed by the human body.
Medical Devices and Skin Absorption Assays
According to EU Regulation 2017/745, medical devices intended for introduction into the human body or applied to the skin and absorbed or dispersed locally are classified based on their absorption characteristics. Class IIa devices are those applied to the skin or in the nasal or oral cavity up to the pharynx, while Class III devices pose the highest risk if they are systemically absorbed or achieve their purpose in the stomach or lower gastrointestinal tract.
Skin absorption assays are typically performed according to OECD428 guidelines, which standardize methodologies and align inter-laboratory data to reduce variability and increase reproducibility through a 3Rs (reduce, replace, refine) approach. These guidelines involve applying a test substance to skin samples in diffusion cells and analyzing receptor fluid samples over time for the test substance and metabolites.
In vitro diffusive models are important for screening the penetration ability of active ingredients in various formulations. These models provide reliable assessment of skin penetration enhancing properties, mechanism of action of carrier systems, and estimation of bioavailability for transdermal delivery. Several in vitro models have been developed to test the penetration kinetics of different compounds across the skin barrier.
Reliable models for reducing or replacing animal tests should be able to recapitulate a biological microenvironment as physiological as possible. Franz Cells are currently used as standard diffusive chambers to perform skin permeation assays, offering a baseline for comparison and validation of alternative methods.
Implications for Pharmacokinetics, Cosmetics, and Regulatory Affairs
The OECD428 guidelines provide general principles for measuring dermal absorption and delivery of a test substance using excised skin. Following these principles, the test substance is applied to the surface of a skin sample separating the two chambers of a diffusion cell. The chemical remains on the skin for a specified time under specified conditions, and the receptor fluid is sampled at various time points throughout the experiment for analysis of the test chemical and/or metabolites. These guidelines outline specific definitions of diffusion cell, receptor fluid, application to the skin, temperature conditions, and sampling modalities, aiming to standardize inter-laboratory protocols and improve the reproducibility of results.
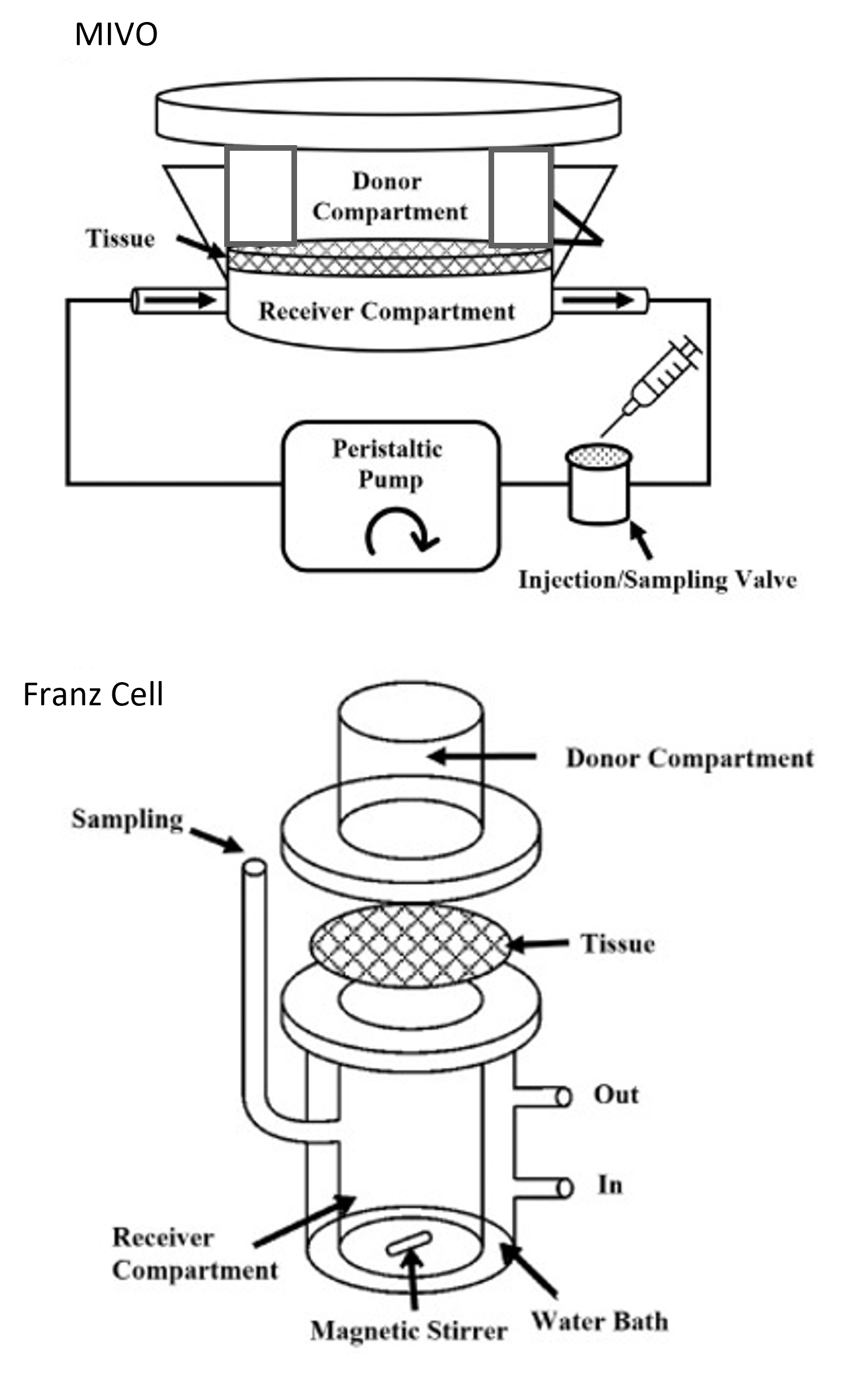
Introducing a Novel Approach with MIVO Technology
MIVO technology presents an innovative alternative to traditional skin absorption assays. It accurately reproduces the three-dimensional complexity of skin tissue and microcirculation, influencing permeation kinetics to closely mimic in vivo conditions. MIVO enables the cultivation of skin cell monolayers, commercially available 3D-reconstructed epithelial tissues, and the use of skin biopsies or artificial membranes as skin surrogates. When connected to a pumping system, MIVO can simulate systemic circulation in vitro, providing precise control over fluid flow rates.
In line with the OECD428 guidelines, the MIVO device consists of a donor and a receiver compartment, physically separated by the skin barrier only, where the skin can be cultured at the air-liquid interface (ALI). The test substance or medical device is applied to the apical side of the skin into the donor compartment, while the basolateral side is in contact with laminar capillary flow. This setup replicates the fluid-dynamic stimuli that physiologically affect the skin, increasing the relevance of the in vitro model and facilitating the absorption of molecules through the skin. The MIVO device features efficient de-bubbling procedures, fast and easy sampling, and high reproducibility, making it an innovative tool for pharmacokinetic, cosmetic, toxicology, and regulatory assays.
Comparison Between Franz Diffusion Cell and MIVO® Technology:
In a study conducted by Pulsoni et al1, we compared Franz Diffusion Cell (FDC) and MIVO technology for in vitro penetration assays using different skin models. The FDC is currently the standard diffusive chamber utilized for skin permeation assays, while MIVO offers a more physiologically relevant environment with its multi-chamber fluidic platform.
The study evaluated the penetration ability of caffeine and LIP1 through the artificial Strat-M® membrane and pig ear skin, serving as human skin surrogates. Both the FDC and MIVO systems exhibited similar penetration kinetic profiles for caffeine and LIP1 upon topical application. However, pig skin tissue displayed a more permissive behavior for lipophilic molecules in the MIVO diffusive system, consistent with in vivo data. This underscores the importance of replicating capillary blood circulation within diffusive systems.
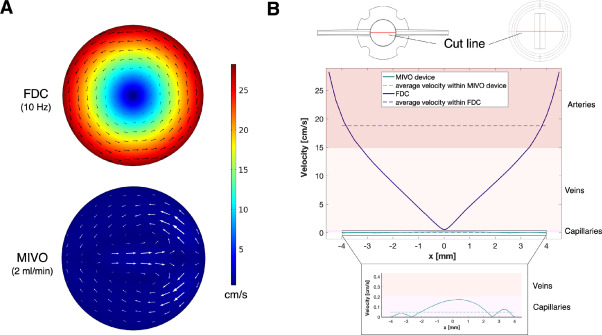
Computational fluid dynamics (CFD) simulations of velocity profiles below the skin were also performed for both FDC and MIVO. A spatially homogeneous velocity profile consistent with capillary blood flow was observed below the skin cultured within MIVO. In contrast, a non-physiological rotational profile was detected within FDC, with velocities ranging from arterial to venous in the outer and inner regions, respectively. This makes MIVO a more suitable diffusion system to recapitulate human blood flow dynamics.
MIVO allows for testing substances on the skin surface while exposing the basolateral side to laminar capillary flow, mimicking physiological fluid dynamics. With efficient sampling, reproducibility, and versatility, MIVO emerges as an innovative tool for pharmacokinetic, cosmetic, toxicology, and regulatory assays.

Reference:
1Ilaria Pulsoni, Markus Lubda, Maurizio Aiello, Arianna Fedi, Monica Marzagalli, Joerg von Hagen, Silvia Scaglione. Comparison Between Franz Diffusion Cell and a novel Micro-physiological System for In Vitro Penetration Assay Using Different Skin Models.
SLAS Technology, Vol 27, Issue 3, June 2022, Pages 161-171Sci

