Skin Toxicity Assay
It is a standard assay for evaluating the toxic biological response induced by test substances topically applied onto the skin. It provides a quantitative assessment of toxicity of medical devices, cosmetics, and drugs, ensuring product safety and regulatory compliance.
Purpose of the Test
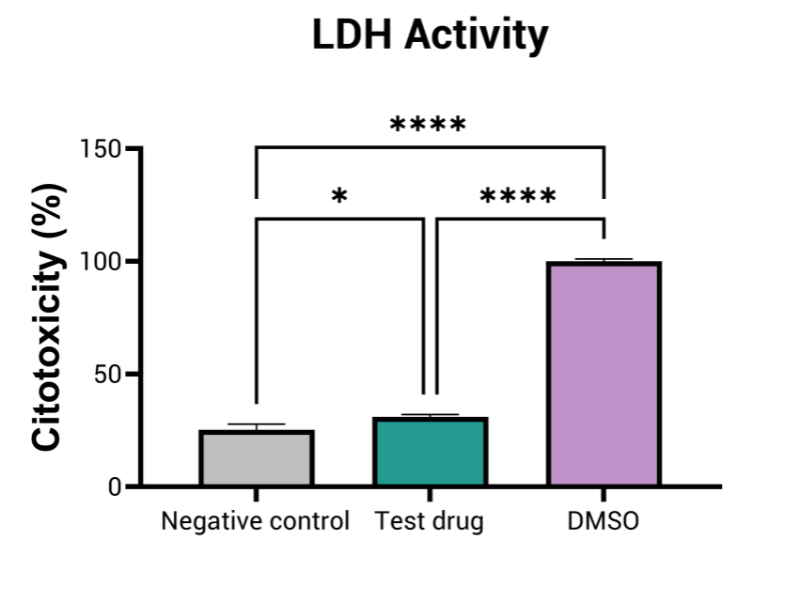
This assay evaluates cell death induced by topical exposure of a compound at different doses, by meauring lactate dehydrogenase (LDH) release, a key marker of citotoxicity.
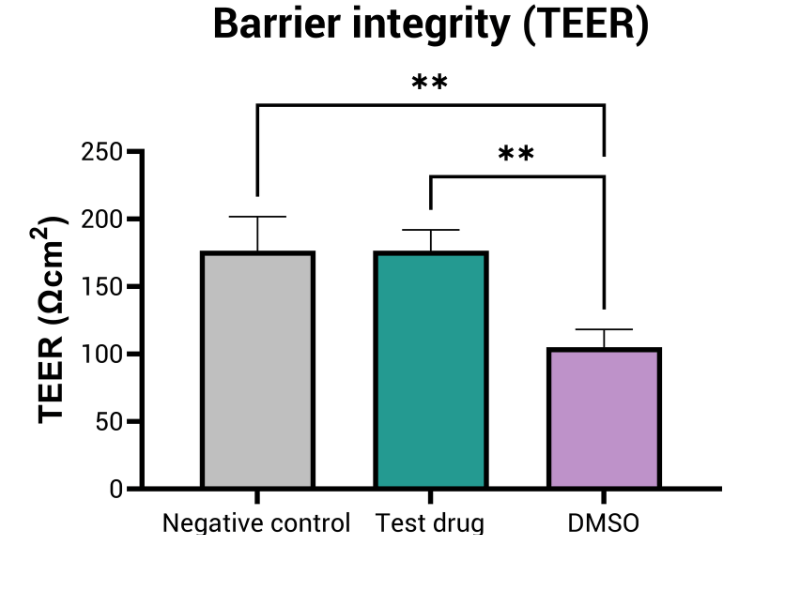
In addition to cytotoxicity, the test assesses barrier integrity variations, providing insights into changes in skin permeability induced by different doses of the compound.
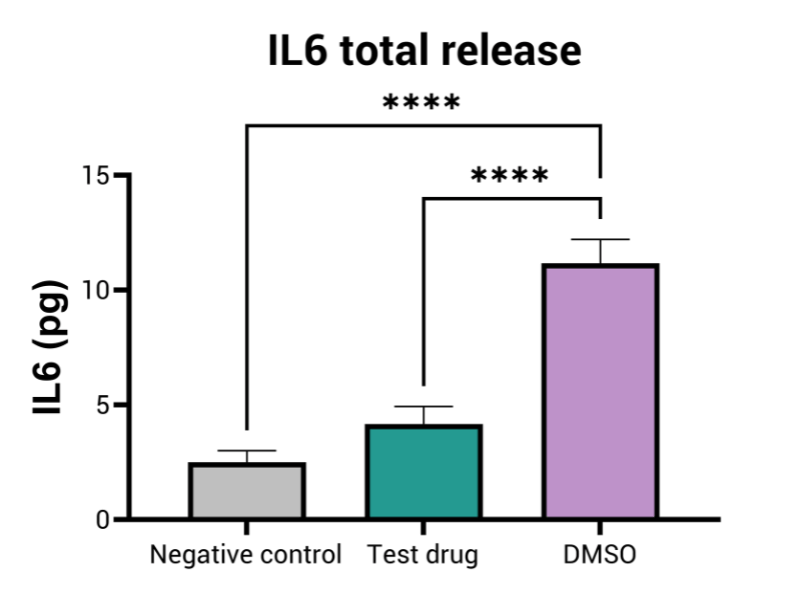
When required, it can also include the analysis of inflammatory cytokines, offering a deeper understanding of biological response to teh tetsing compounds.
These evaluations are essential for regulatory safety assessments in product development and preclinical drug testing.
The MIVO® Advantage: A Superior Predictive Model
The MIVO® Skin-on-Chip platform enhances conventional cytotoxicity testing by incorporating physiological fluid flow beneath the skin tissue, creating a biomimetic environment that better replicates human physiology. This dynamic system provides a more precise and reliable evaluation of compound effects, not only in terms of toxicity but also regarding skin barrier function and inflammatory responses. By offering a more comprehensive assessment, it improves the predictability of in vitro results, supporting the development of safer and more effective biomaterials, drugs, medical devices, and
cosmetics.

Our Unique Value Proposition
Skin Toxicity Assay
In summary
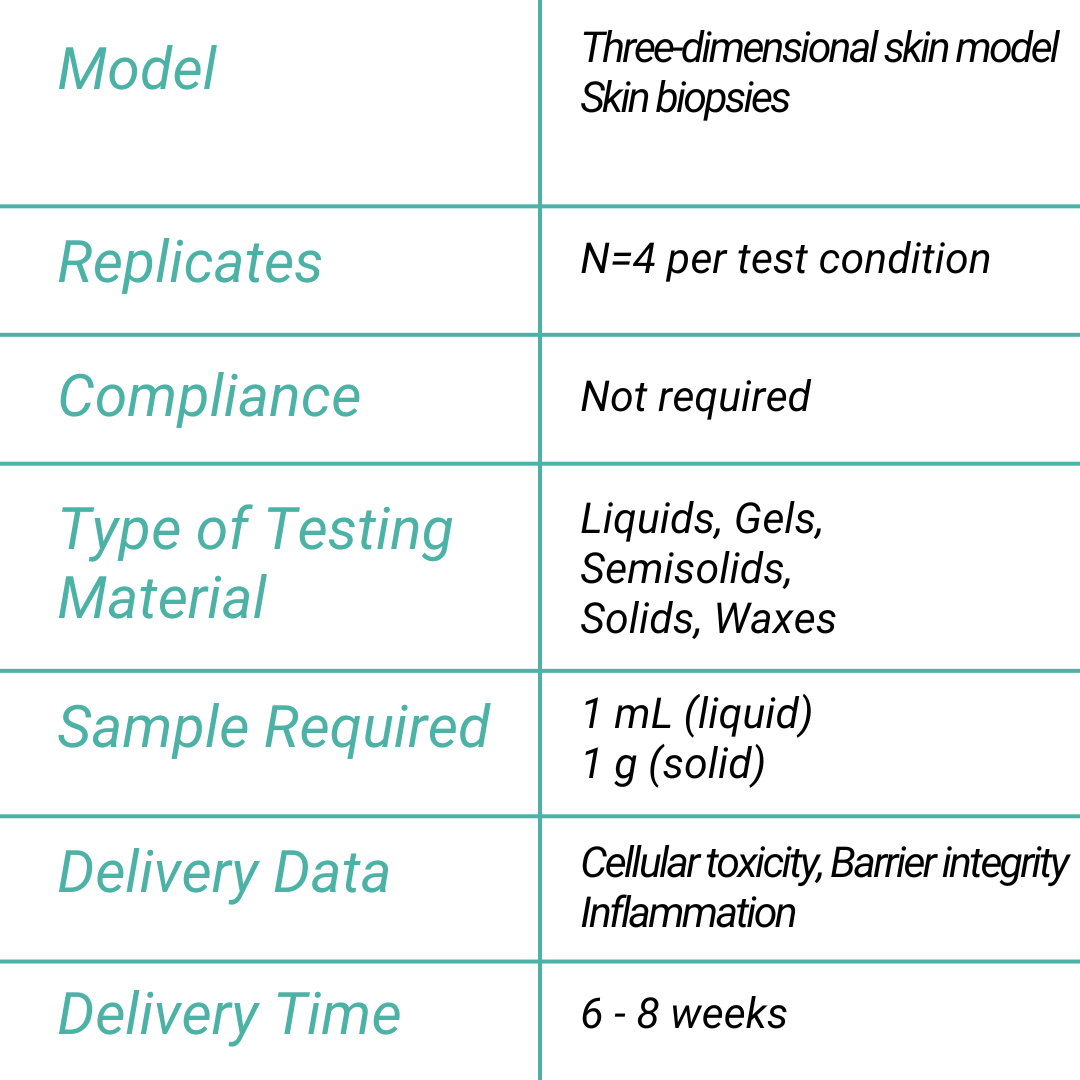
READOUTS
- Cellular toxicity (i.e. LDH activity)
- Epithelial barrier integrity (TEER measurement)
- Inflammatory cytokines profiles (optional)
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.