Skin Irritation OECD439
Skin irritation assay is essential in product development and regulatory compliance, to ensure the safety of substances coming into contact with human skin, being crucial for cosmetics, pharmaceuticals, and chemicals.
Purpose of the Test
The MIVO-based skin irritation assay uses the MIVO® skin-on-Chip platform to evaluate the safety of testing substances in a more reliable and human-relevant manner than traditional in vitro models. MIVO skin-on-Chip platform ®’s ability to faithfully recreate the complex dynamic fluid-flow structure of the human skin, along with its compatibility with Reconstructed Human Esophageal (RhE) tissue models, allows to mimic human skin structure
and function and predict skin responses to test substances.
The MIVO® Advantage: A Superior Predictive Model
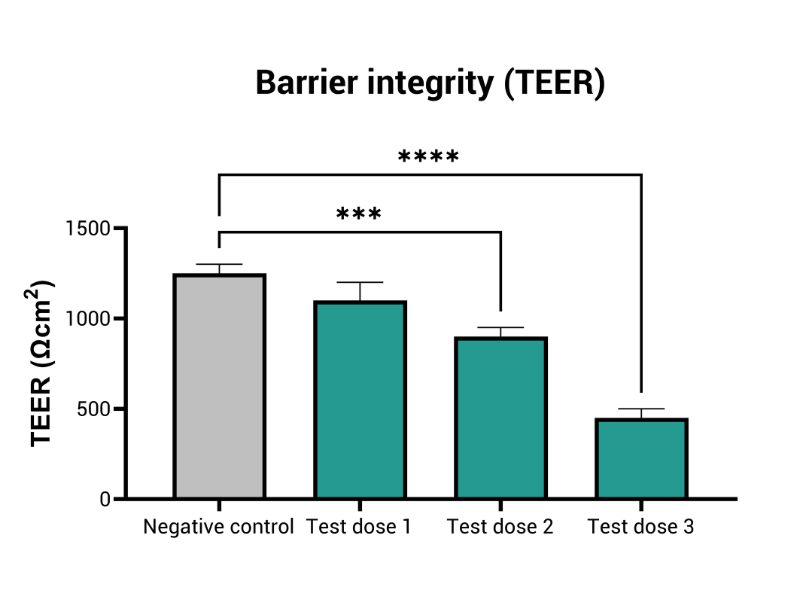
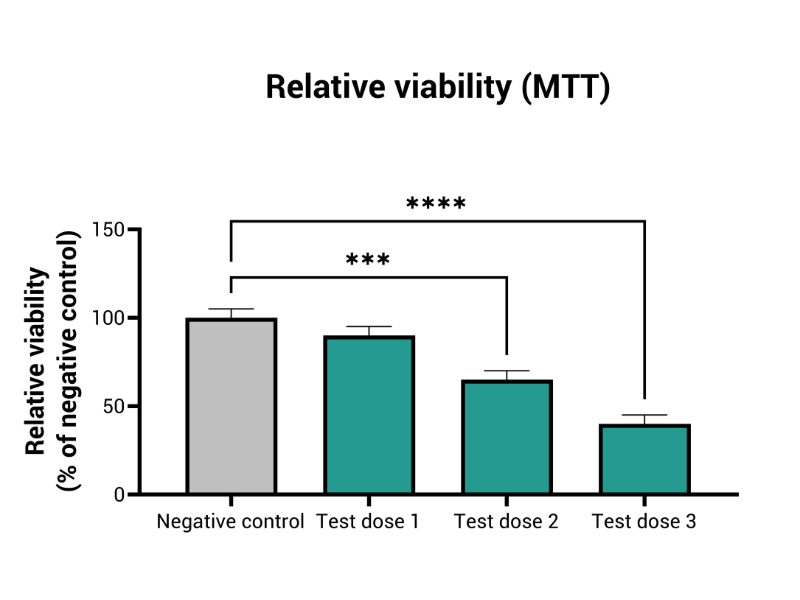
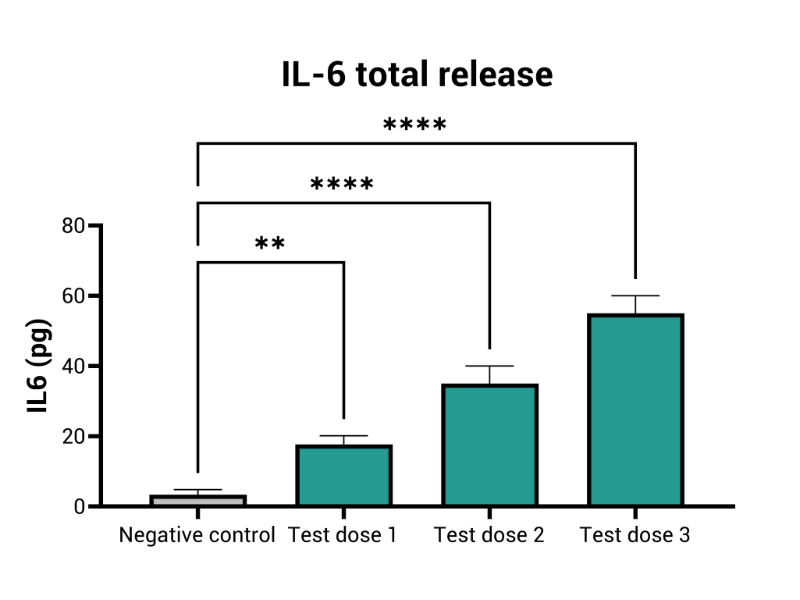
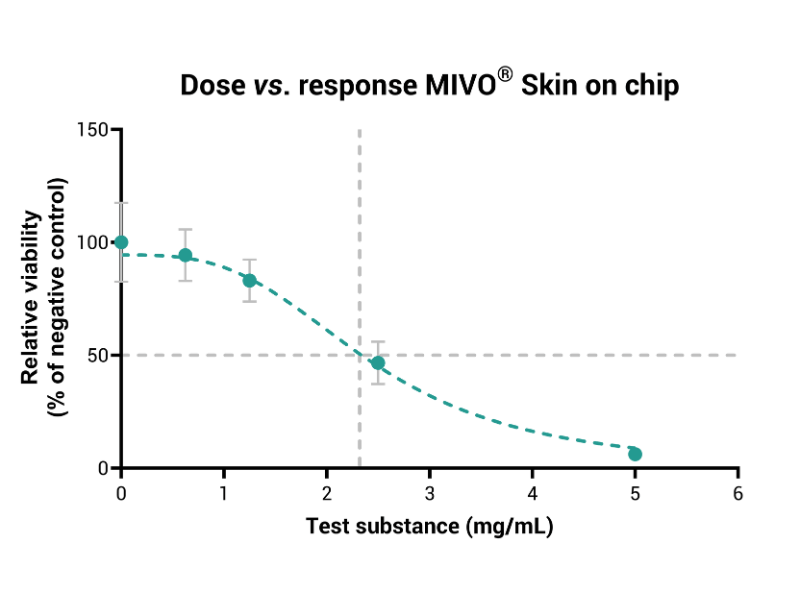
MIVO® organ-on-chip technology enhances traditional RhE models by introducing dynamic fluid flow, creating a more accurate simulation of physiological skin conditions. This dynamic interaction improves the prediction of local skin toxicity, providing a comprehensive, accurate solution for skin irritation testing.
Our Unique Value Proposition
Skin irritation assay
In summary


READOUTS
- Epithelial barrier integrity (TEER measurement)
- Tissue viability (MTT) and EC50 evaluation
- Inflammation (i.e. IL6, IL8)
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.