The most widespread models used by basic researchers and pharmacological companies can be divided into two categories: two-dimensional (2D) cell cultures for the in vitro approach and the use of animal models for the in vivo one.
However, the current employment of 2D cultures or animal experimentation remains unsatisfactory as these models cannot provide an efficient and accurate platform for studing molecular mechanisms and human cell behaviour behind multiple diseases or predictive preclinical evaluation of new therapies and drugs.
The 2D cell cultures, despite having the advantage of being highly reproducible, robust and low cost, also have the great limit of proposing an approach that results too minimalist in a research world that aims to models that are increasingly physiological and comparable with the in vivo situation.
Nowadays, results obtained from traditional 2D culture can be only considered preliminary, both in drug testing for efficacy and toxicity and in unravelling molecular pathways underlying cellular behaviour or pathologies. It is extensively demonstrated that forcing cells to adapt and grow on a flat, rigid surface affects essential cellular mechanisms such as their proliferation, differentiation, functionality and response to stimuli.
All these behaviours are altered by the lack of physiological complexity and cell-cell and cell-environment interactions that are intrinsically present in our organs and bodies.
On the other hand, the use of animal models, while providing a more systemic and comprehensive point of view of molecular pathways and drug responses, remains an alternative platform. It implies not only ethical problems and high maintenance costs, but above all, species-specific differences that make them poorly predictive and reliable in a pharmacological context.
80% of potential therapeutics fail in clinical trials although their proven efficacy and safety in animal models, which despite all, is still the golden standard in preclinical studies.
Trying to take the best of both these models and aiming to create more physiological ones has led in the recent years to increase the applications of three-dimensional (3D) cell cultures.
They can better recapitulate cellular complexity until recreating an organ functionality in vitro, helping to design a physiological-resembling extracellular environment.
This feature is essential in cancer research, where the possibility to reproduce an in vivo-like tumour microenvironment (TME) while maintaining a simple way of cultivating cells and adaptability of protocols already validated on 2D cultures is crucial.
It has been proven that TME has a critical role in tumour initiation, development, and in processes like tumour escape from the immune system and metastasis. 3D cultures allow tailoring the extracellular matrix (ECM) based on the cell types used, which is a significant advantage while studying crosstalks.
The most popular 3D models used in laboratories all over the world:
• Organoids, obtained through the use of different kinds of matrices;
• Spheroids, which are self-assembled 3D tumour cultures;
• Polymeric scaffolds, which are porous substrates that can support cell growth within their architecture;
• Reconstructed tissue, even with the aid of 3D printing technique;
• Patient-derived biopsies.
The use of 3D cultures allows cells to grow, differentiate and be responsive to stimuli in a way that much more resembles their physiological behaviour in vivo, including their proliferation, genes and proteins expression, migration or drug resistance.
The introduction of fluid-dynamic stimuli to the 3D cells culture remarkably improves the relevance of in vitro models, leading to a deeper investigation of cellular processes such as cell migration, infiltration, cancer cells metastasis, cancer-immune cells crosstalk and providing a more reliable platform for testing novel therapies and drugs.
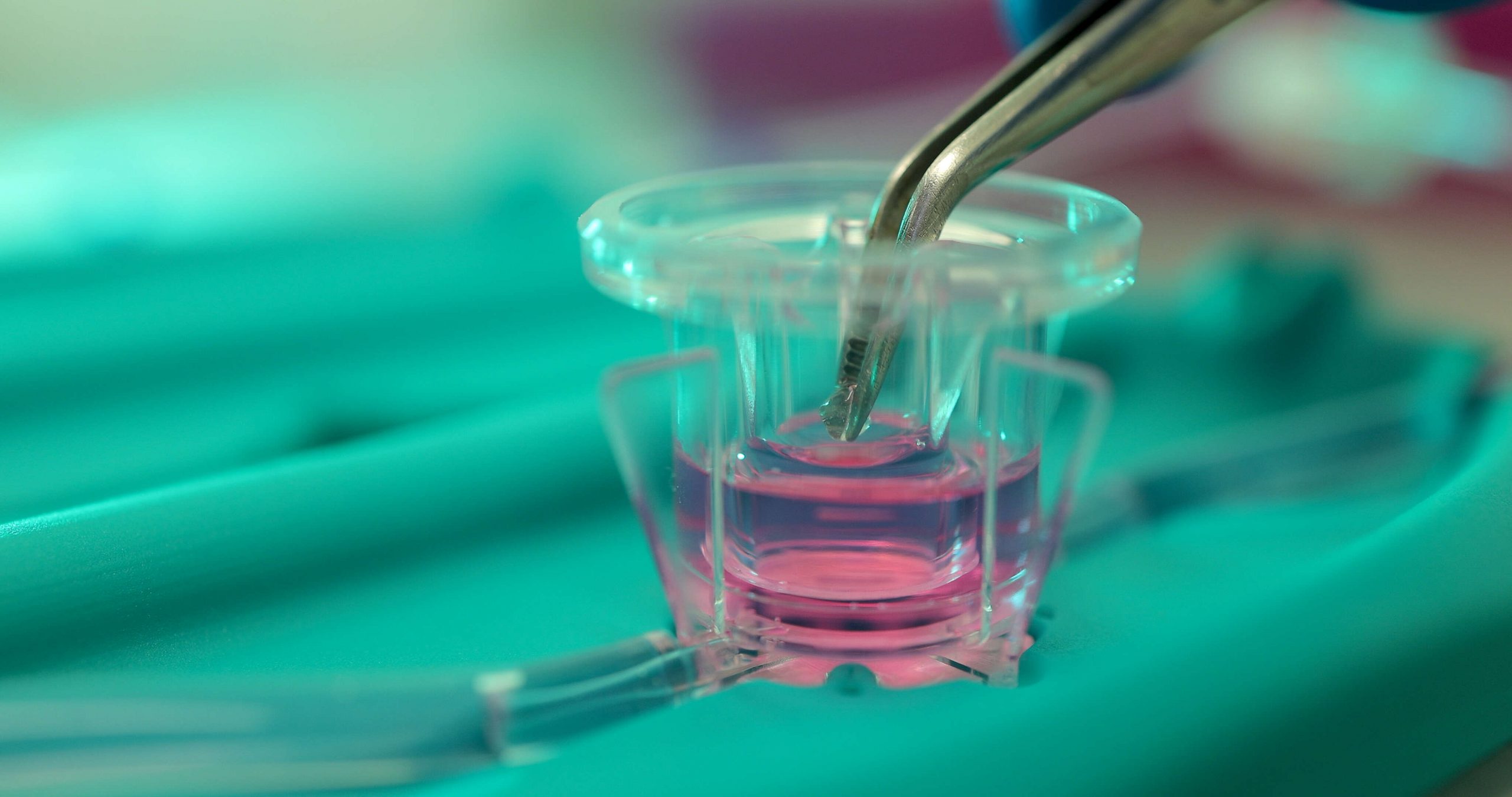
In this context, our patented MIVO® technology is a reliable fluid dynamic platform composed of an optically transparent cell culture chamber compatible with the commercially available 24-well plate’s size inserts.
Connecting the MIVO® device to a peristaltic or syringe pump, it is possible to mimic the systemic circulation in vitro in a highly controlled model, culturing clinically relevant size cancer tissues and patient-derived tissue models under physiological flow conditions.
Multiple chambers can also be fluidically connected to set up a multi-organ platform suitable for different studies like (i) recapitulating the overall journey of cancer cells to their metastatic sites, (ii) reproducing the physiological environment of immune cells to investigate their behaviour or (iii) exploring the ADME of new drugs in different tissues simultaneously, in a more physiological way.
To learn more about MIVO® Platform click here.

