Leaky Gut Assay
The integrity of the intestinal barrier is crucial for maintaining overall gut health, preventing harmful
substances from entering the bloodstream. When this barrier is compromised, a condition known as “leaky gut” occurs, which is associated with gastrointestinal disorders and systemic inflammation.
Purpose of the Test
This assay evaluates the regenerative potential of a test substance by tailoring an advanced human leaky gut in vitro model. By creating a controlled wound in the gut
cells monolayer, the assay measures wound closure rates and barrier function over time, providing valuable insights into the substance’s ability to restore epithelial integrity and promote gut barrier repair.
This assay is generally used as a standard in preclinical studies to support the safety, efficacy, and therapeutic claim of a product.
The MIVO® Advantage: Accelerating Epithelial Repair Insights
The MIVO® platform offers a groundbreaking approach to studying leaky gut by simulating both luminal and blood flow in a dynamic, human-relevant gut environment. Unlike traditional static models, which fail to replicate the complex physiological conditions of the human gut, MIVO® advanced organ-on-chip system allows for more accurate assessment of epithelial barrier restoration and gut permeability in real-time. This innovative model enables researchers to evaluate the ability of test substances to repair leaky gut more effectively, providing a predictive tool for understanding therapeutic efficacy.
Driving Innovation and Setting New Standards
While regulatory frameworks for probiotic testing in gut health are still evolving, the MIVO® platform positions research at the forefront of this emerging field, paving the way for a new era in microbiome research. This innovative approach challenges the status quo, providing critical data that can drive further scientific exploration and potentially shape future regulatory standards in probiotic and microbiome-based therapies.
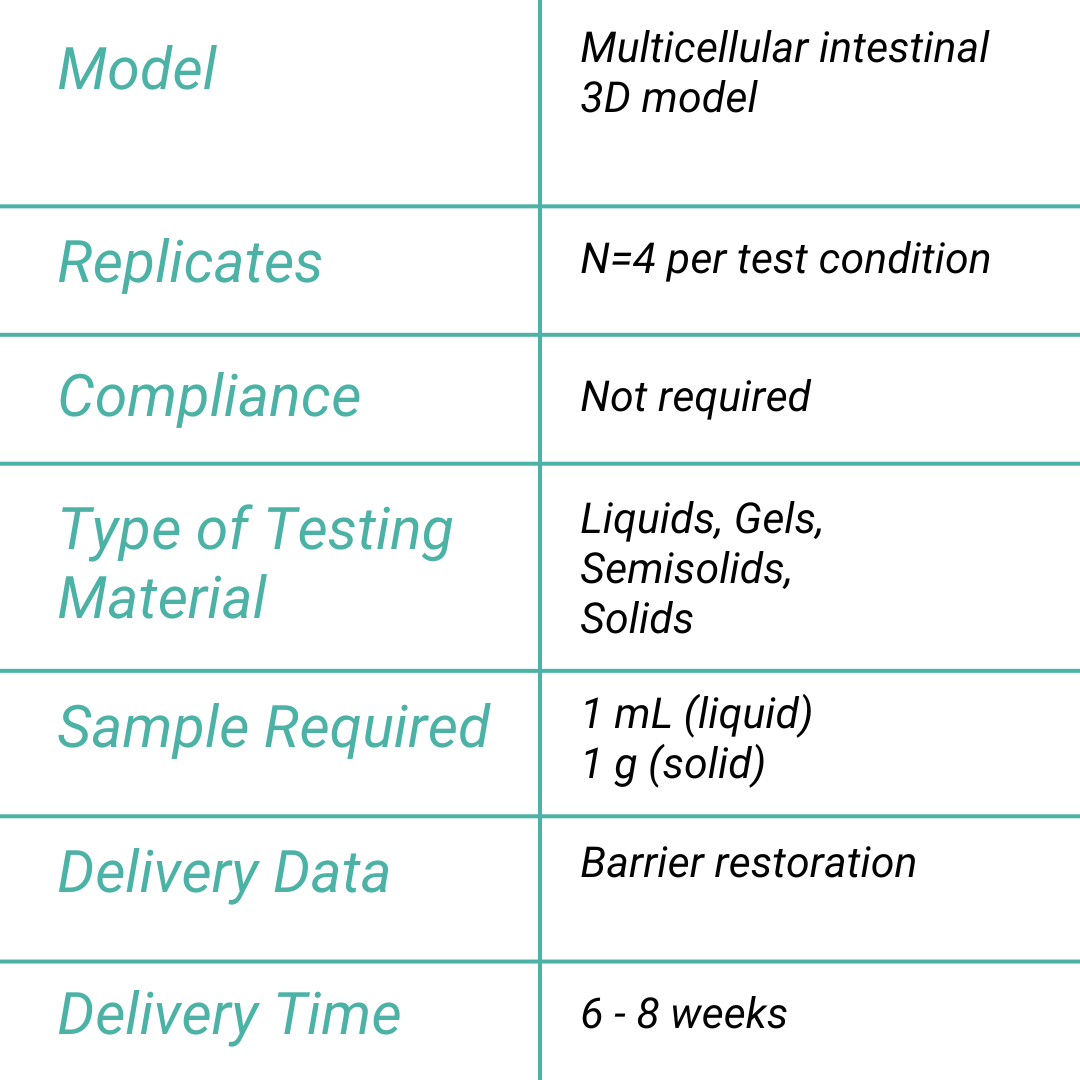
Our Unique Value Proposition
Leaky Gut Assay
In summary
READOUTS
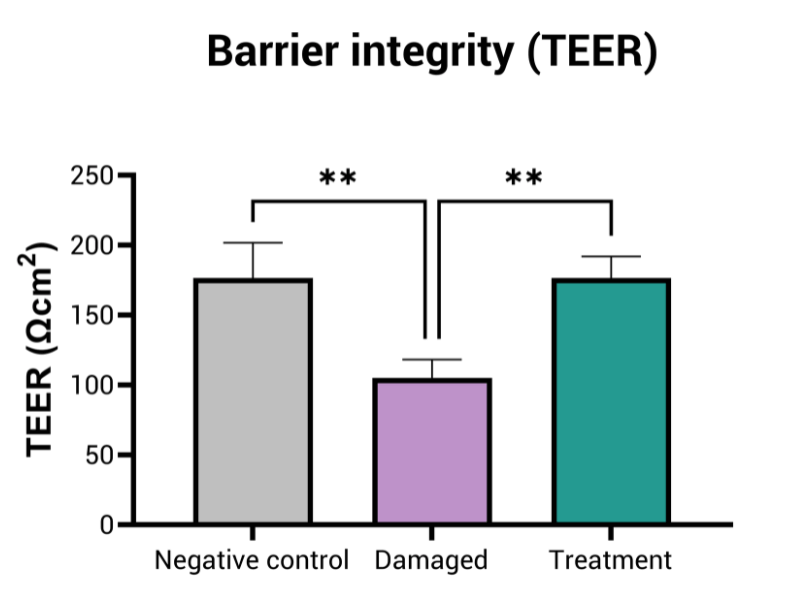
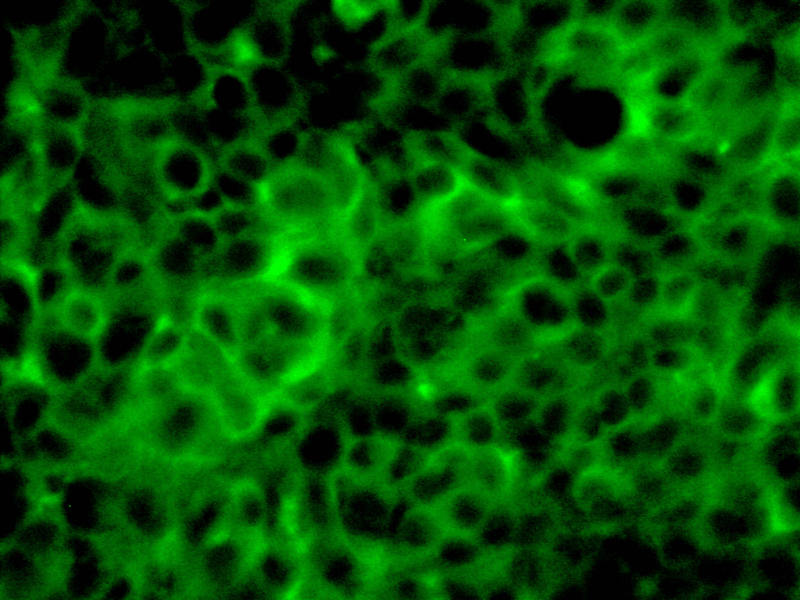
- Epithelial barrier integrity (TEER measurement)
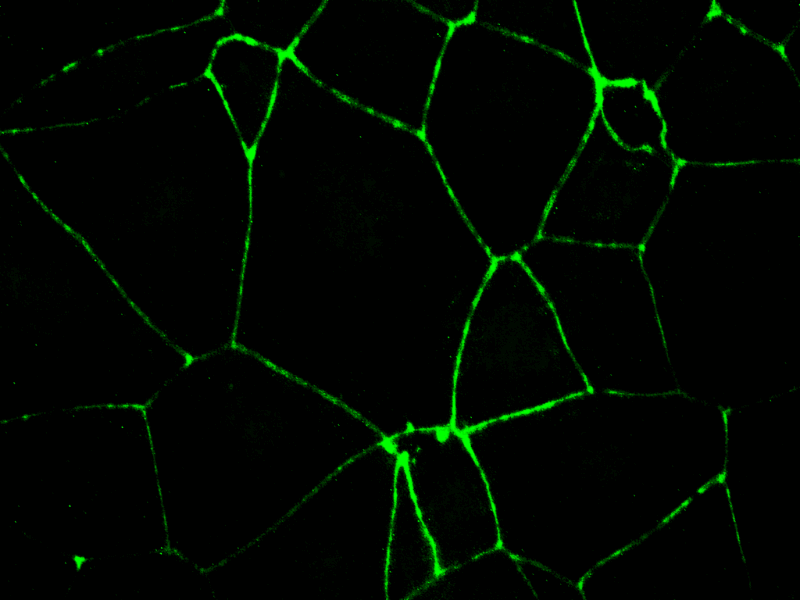
- Tight junctions specific staining
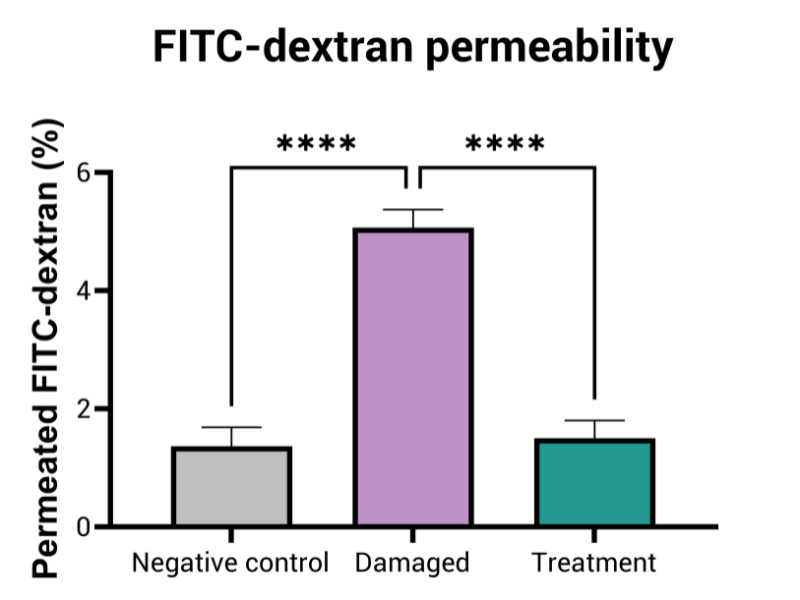
- FITC-dextran permeability
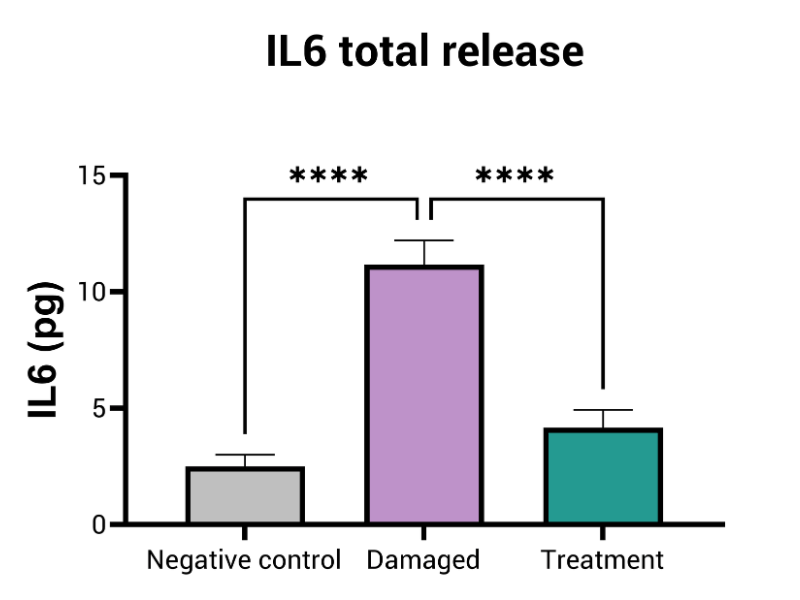
- Inflammation (i.e. IL6, IL8)
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.