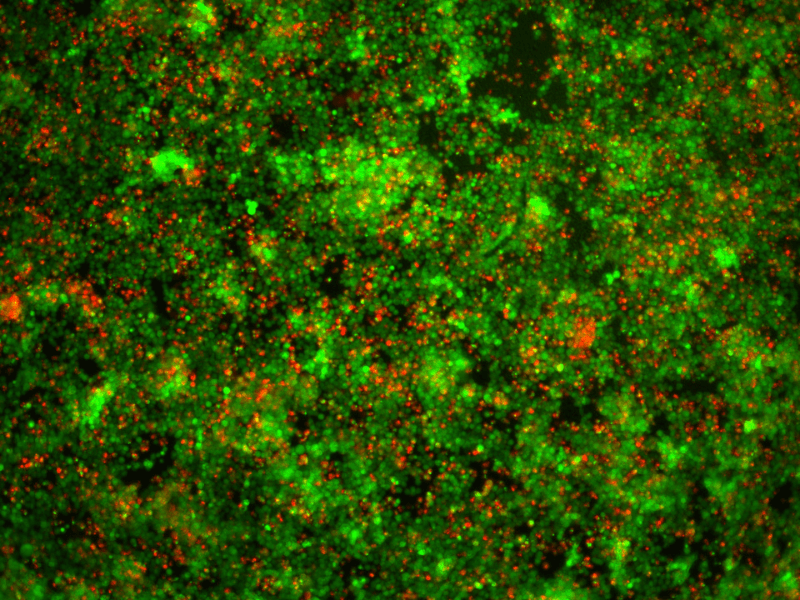
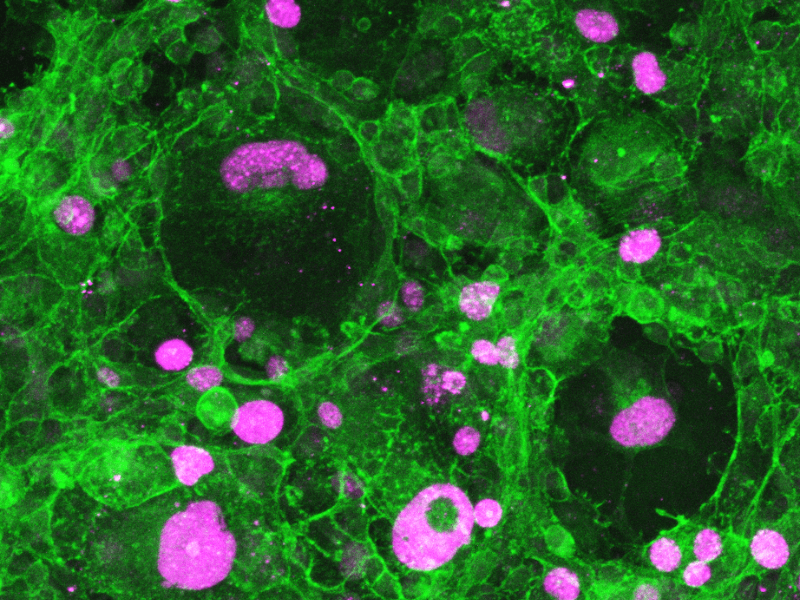
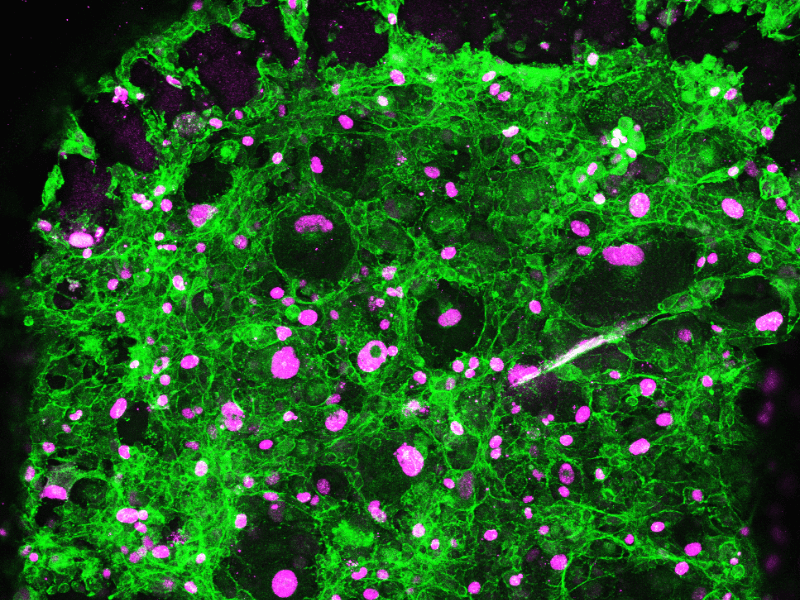
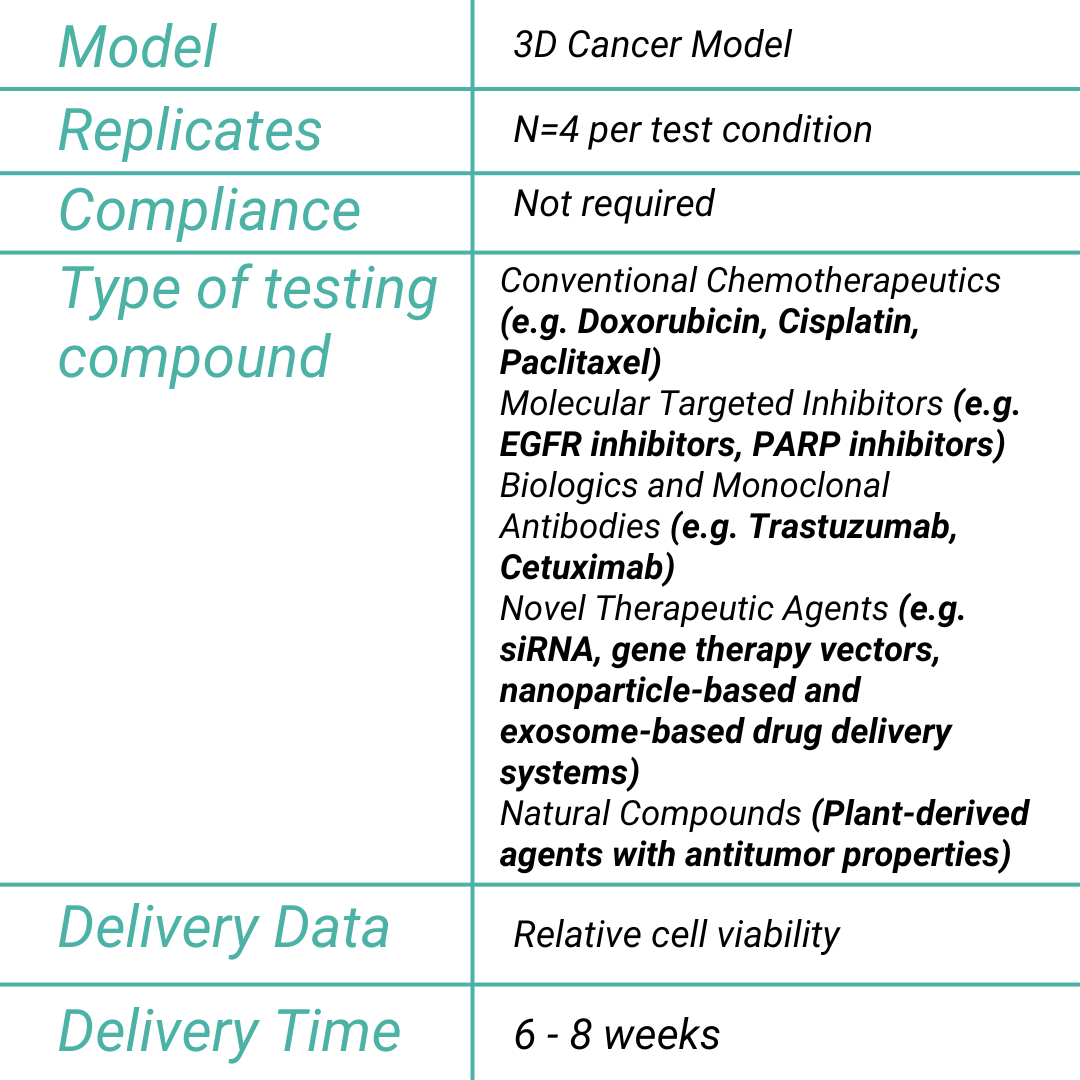
Drug Efficacy Assay
The Drug Efficacy Assay evaluates the therapeutic potential of new anticancer drugs by using three-dimensional human cancer models, ensuring early detection of antitumor activity critical for drug development.
Purpose of the Test
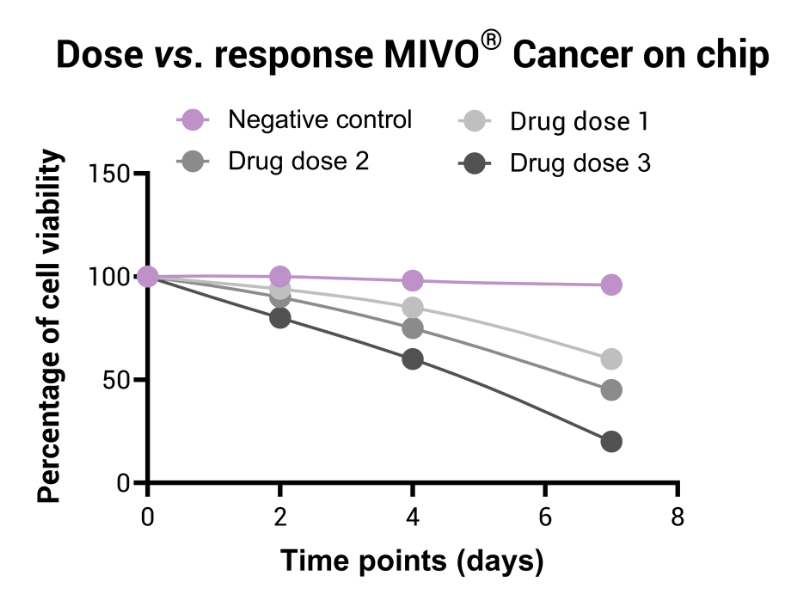
By employing advanced in vitro clinically relevant cancer models that replicate the complex architecture and microenvironment of tumors, this assay assesses the efficacy of new therapeutic compounds administered via the cancer on chip platform. The drug circulation perfectly mimics the systemic drug delivery, allowing robust monitoring of drug-induced reductions in tumor viability via omics and histological analysis.
In alignment with recent guidelines from regulatory bodies such as the FDA and EMA, this assay is instrumental in preclinical evaluation. It enables rigorous assessment of therapeutic efficacy, ensuring that promising drug candidates are thoroughly characterized before advancing to clinical trials, thereby reducing reliance on animal models.
The MIVO® Advantage: A Superior Predictive Model
This innovative platform enhances traditional static assays by integrating dynamic fluid flow and 3D cancer models to simulate systemic drug administration and proper penetration into the tumor matrix. The continuous circulation improves drug transport and distribution within the 3D tumor model, yielding efficacy data that closely correlate with in vivo outcomes (ref drug efficacy).
Our Unique Value Proposition
Drug Efficacy Assay
In summary
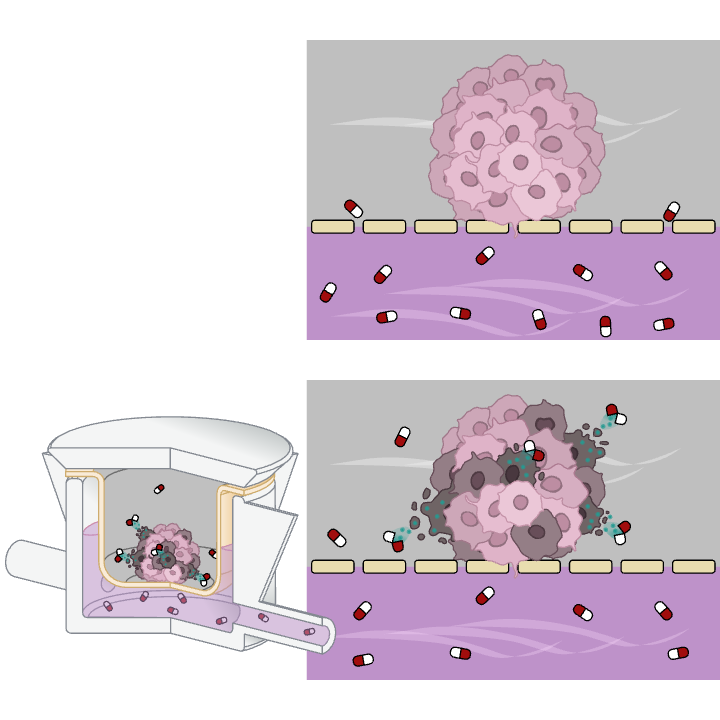
READOUTS
- Cell viability (Live/Dead)
- Relative cell viability (MTT or AlamarBlue)
- Tumor cell proliferation and behavior (ki67 staining)
- Hystology (optional)
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.