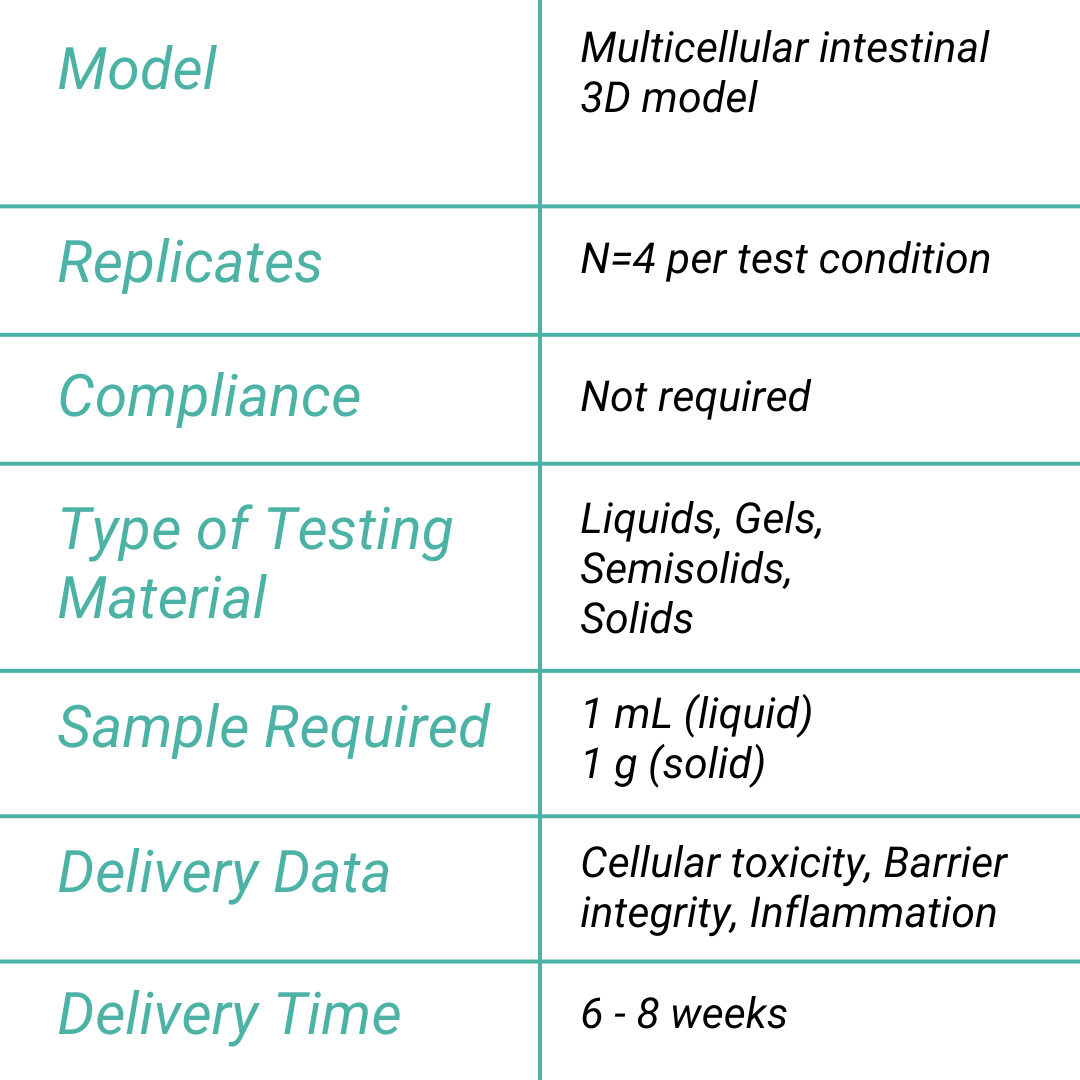
Drug Induced Toxicity Assay
The Drug-Induced Intestinal Toxicity Assay evaluates the potential harmful effects of drug candidates on gastrointestinal (GI) tissues, ensuring early detection of adverse reactions that could compromise drug safety.
Purpose of the Test
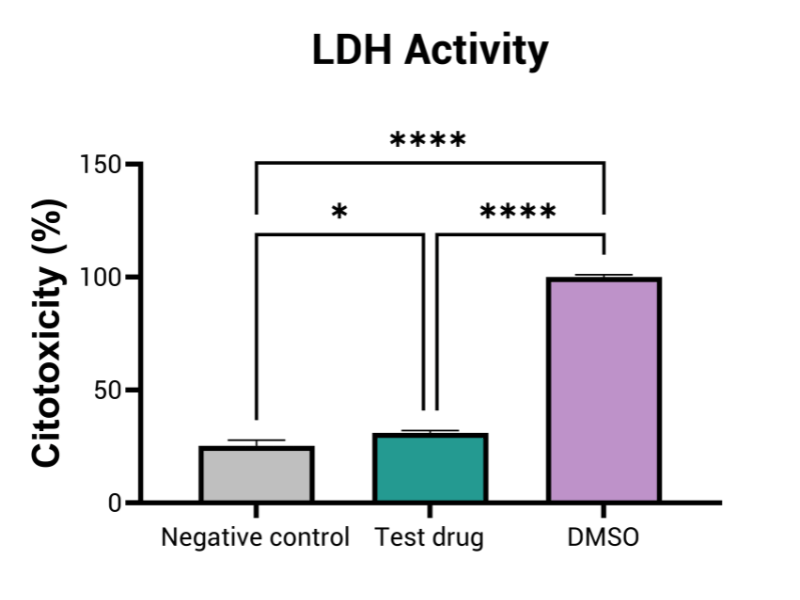
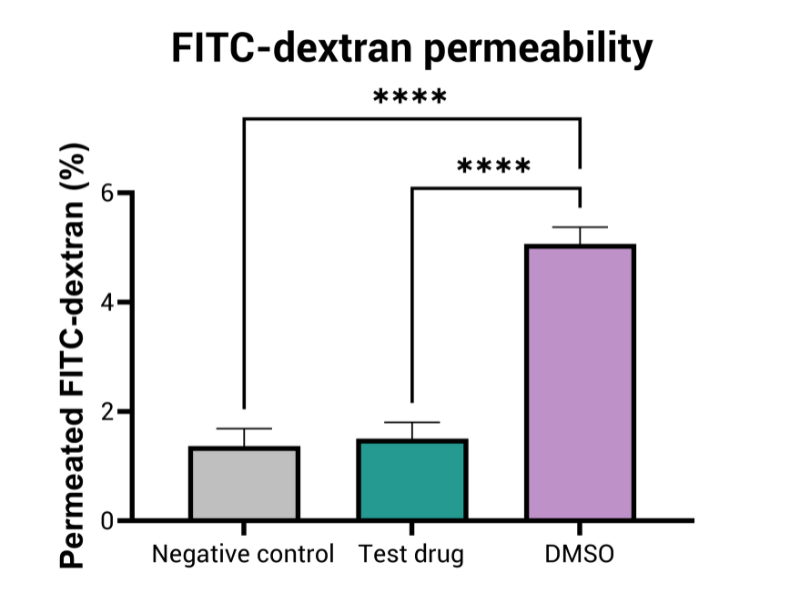
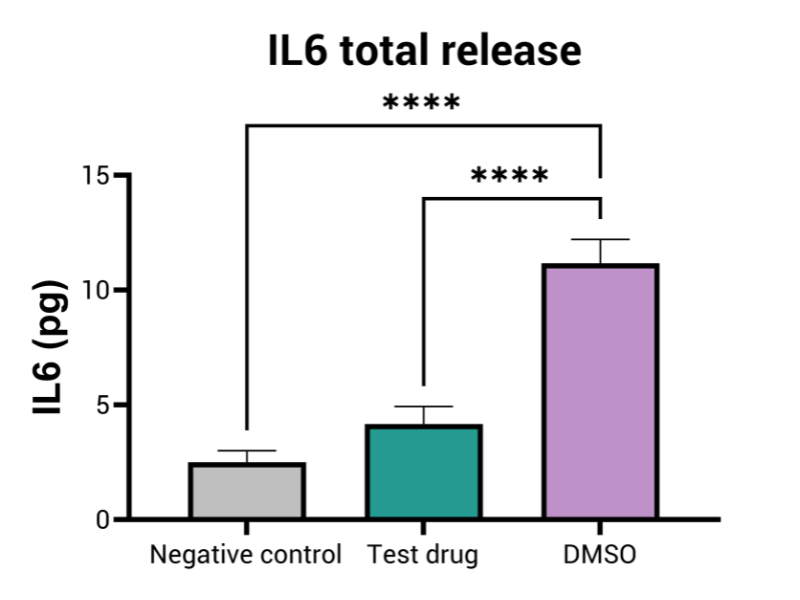
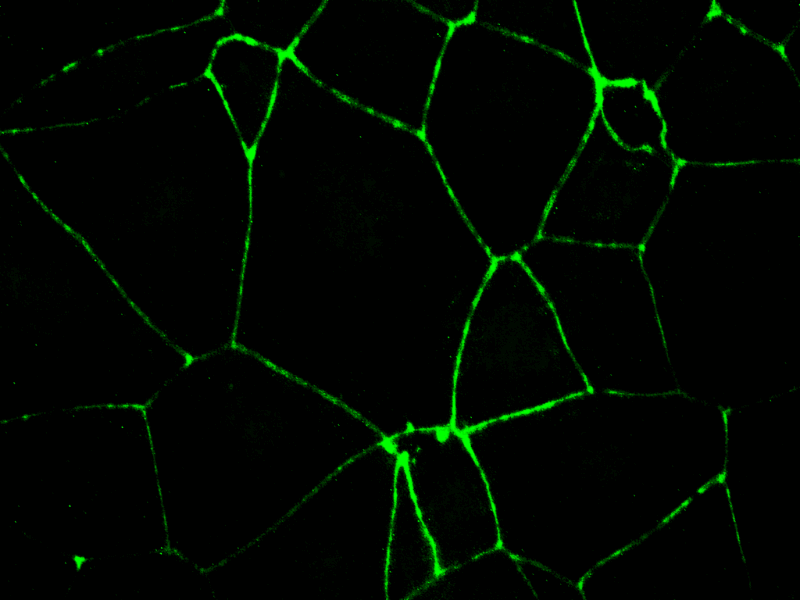
By employing advanced in vitro models that mimic human gut physiology, this assay identifies GI toxicities, such as inflammation, barrier disruption, mucus alteration, and tissue damage. These models may integrate multiple cell types—including epithelial, immune, and bacterial cells—providing a more comprehensive representation of the GI tract’s complexity and function.
Aligned with the requirements of regulatory bodies, this assay is vital in predicting gastrointestinal toxicity of drugs at different doses. It plays a crucial role in the submission process for new drug candidates, contributing to the development of safer pharmaceuticals and minimizing the chances of GI complications in clinical trials.
The MIVO® Advantage: A Superior Predictive Model
The MIVO® platform elevates traditional GI toxicity assays by introducing dynamic fluid flow, simulating blood circulation and luminal conditions to better replicate in vivo GI environments. Unlike static in vitro models, MIVO® allows for real-time assessment of drug interactions, providing a more accurate evaluation of drug-induced toxicity. This dynamic culture system enhances the physiological relevance of the assay, offering improved predictions of gastrointestinal responses.

Our Unique Value Proposition
Drug-Induced Toxicity Assay
In summary
READOUTS
- Intestinal Cellular toxicity
- Epithelial barrier integrity (TEER measurement)
- Tight junctions specific staining
- FITC-dextran permeability
- Inflammation (i.e. IL6, IL8)
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.