SKIN Permeation Assay OECD428
Understanding the permeability of substances through the skin is crucial for evaluating the safety and classification of topical products, pharmaceuticals, medical device and cosmetics. Skin permeation assays provide valuable insights into compounds absorption, delivering essential data for product development, repurposing, and regulatory approval.
Purpose of the Test
The skin permeation assay evaluates the fraction of test
substances absorbed using advanced in vitro or ex vivo skin models cultured within an innovative MIVO based diffusive chamber, which mimics in vivo conditions.
Regulatory Compliance
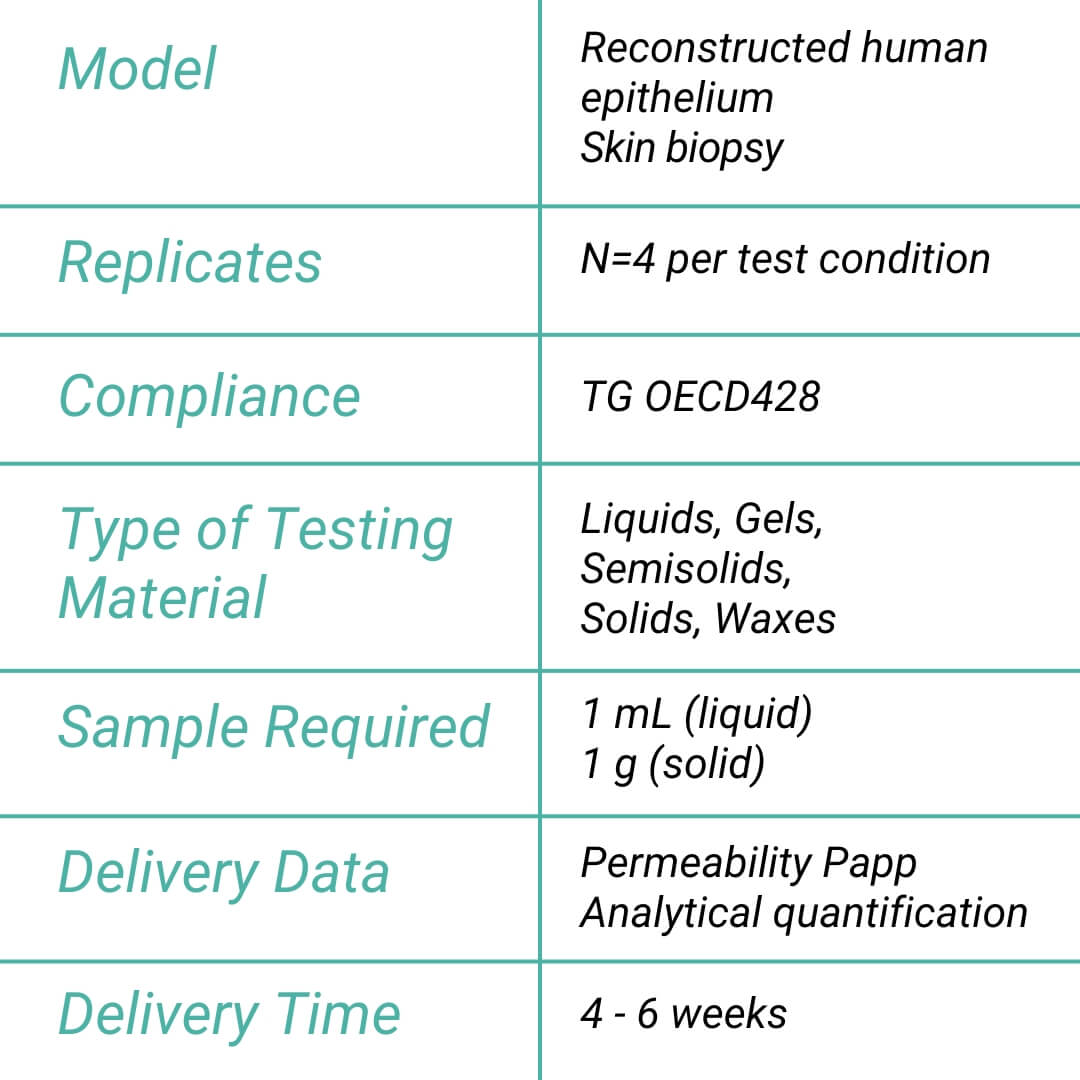
This assay adheres to the OECD Test Guideline 428, ensuring that products are tested and compliant with global safety standards.
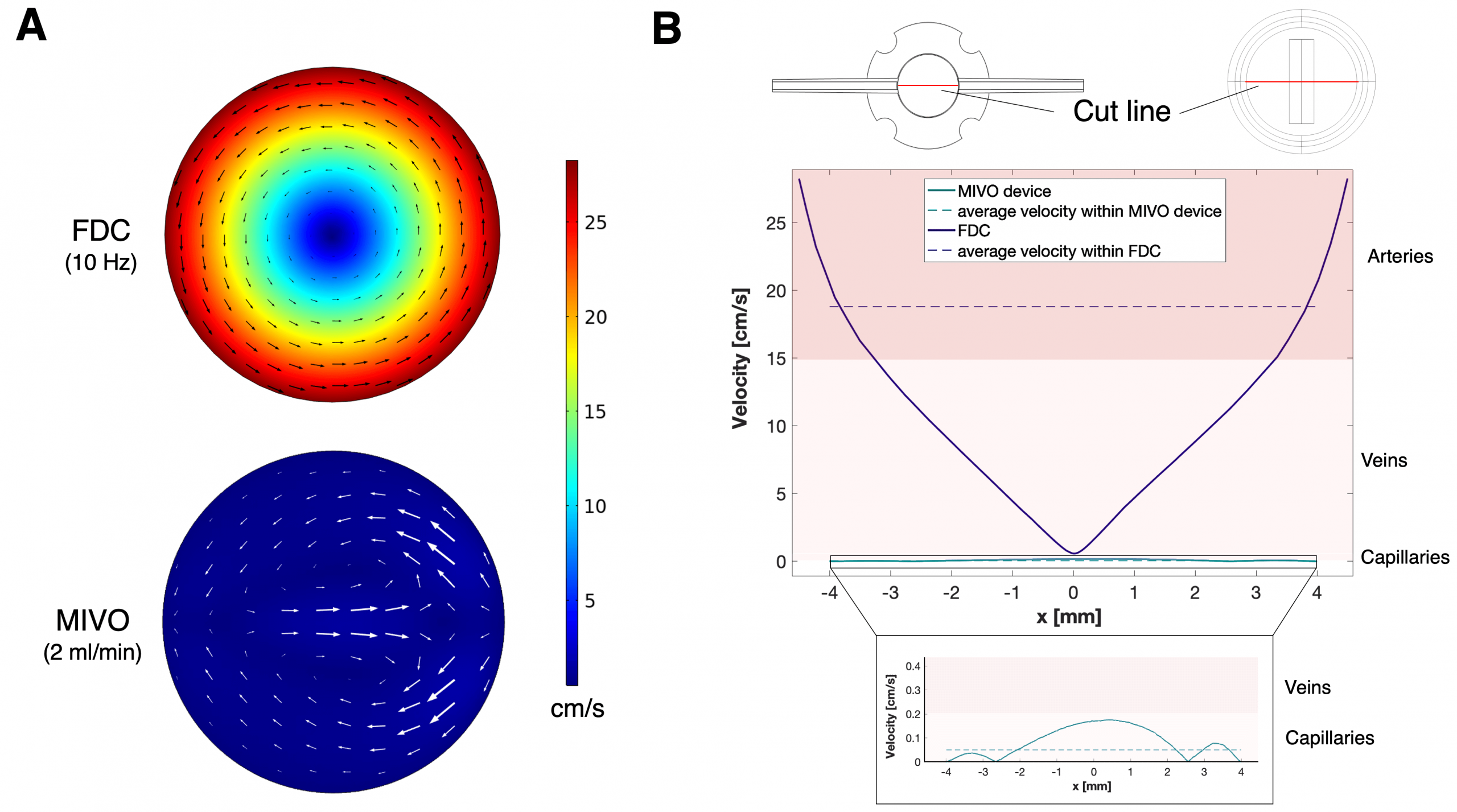
The MIVO® Advantage: A Superior Predictive Model
MIVO® skin on chip technology provides an advanced platform for evaluating skin permeability by closely replicating the physiological conditions of human skin. By introducing dynamic fluid flow, MIVO® enables more accurate measurements of substance absorption and skin penetration, offering reliable insights into product performance and safety.
Our Unique Value Proposition
Skin Permeation Assay
In summary

READOUTS
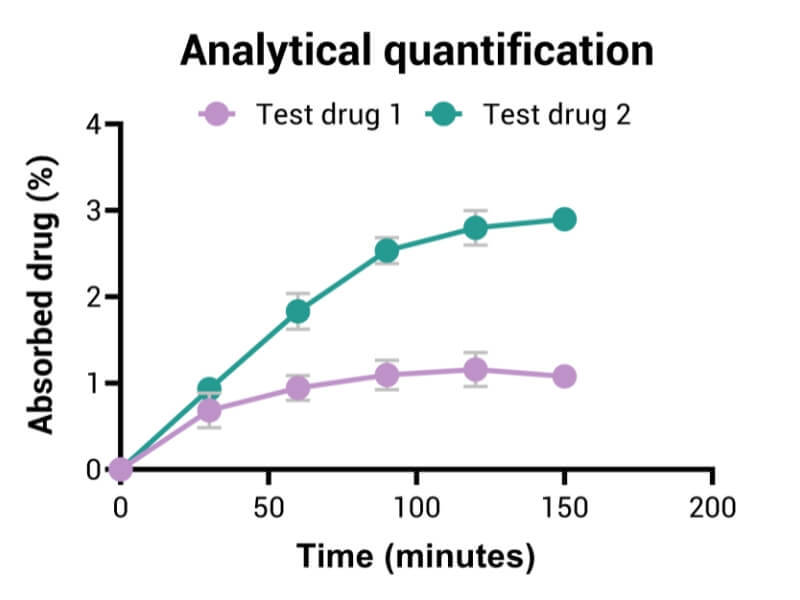
- Analytical quantification of kinetics of the fraction of molecules absorbed
- Measure of apparent permeability coefficient (Papp)
Get a Quote
We are here for you
Whether you’re looking for a quote or want to connect with us, fill out the form below with your details, and we will be in touch shortly.
All fields are required, take your time.