The technology allows us to use the so-called organoids, three-dimensional (3D) structures formed by the cells extracted from the patient himself, for in vitro tests aimed at identifying the most effective drug.
Everyone is talking about personalized medicine very often, but what exactly does it mean, and how could it be possibly implemented? The model of personalized medicine proposes the personalization of the therapy, and therefore, the use of medical decisions and drugs that are specifically selected for the patient. In fact, each person is unique, and therefore his therapy must also be so. Science has shown that cancer is like a set of hundreds of diseases, each one with unique characteristics and genetic profile, and that’s why we can identify over 300 types and subtypes of cancer. One way to establish the best cure for each patient is to use the so-called organoids, three-dimensional (3D) entities formed by cells deriving from the patient himself, to be used, for example, for in vitro tests that could determine the most effective drug. Specifically, organoids are 3D cellular structures equipped with an extracellular matrix that lead to a simplified, and sometimes miniaturized, version of a human organ. Precisely, as these organoids are similar to their organs of origin, they are promising tools in medical research and in the development and testing of new treatments: their therapeutic potential is useful in identifying specific cure and in providing real personalized medicine for individual patients.
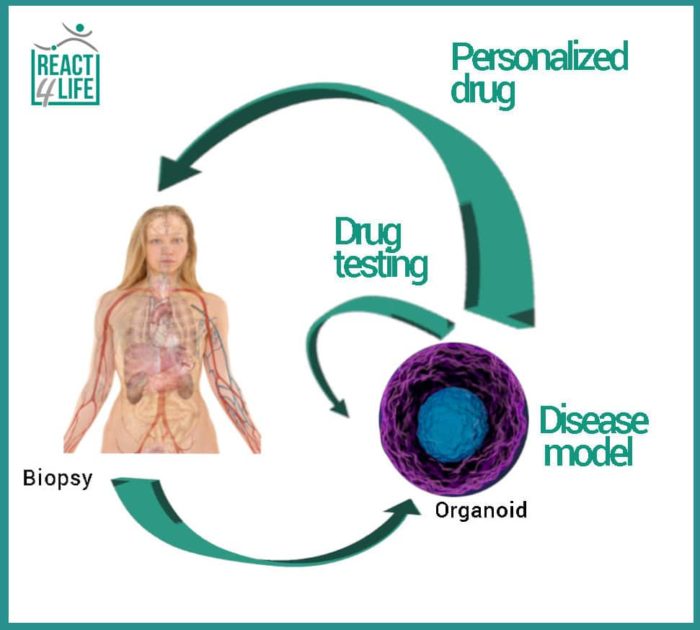
Grown from patient cells organoids are mainly used as a disease model: in this way, our knowledge of the biological mechanisms underlying certain pathologies would be enhanced. Moreover, organoid-based assays can integrate, or even reduce, the current animal experimentation in testing the efficacy of new molecules. In the next future, these patient-derived organoids will help researchers to identify the ideal treatment as it is specific for each patient.
As regards the research focused on tumors, scientists are currently employing three main systems as cancer model: two-dimensional (2D) culture, xenografts and organoids. 2D cell culture refers to the culture of cancer cells monolayers on plastic substrates. The main disadvantage of this technique is its inability to faithfully reproduce the clinical spectrum of the cancer, due to an unrealistic cell-cell interaction forced to be developed in a plane rather than in a three-dimensional space, as in the in vivo condition.
A second approach involves the transplantation of human tumor cells into immunodeficient mice (xenotransplantation), thus obtaining an in vivo model, certainly more realistic than the in vitro 2D one, where the heterogeneity of the tumor is preserved. However, this model appears to be cost and time-consuming, and not always predictive in terms of the tumor propagation and the response by the organism, since the differences between the mouse and human species remain significant.
The use of organoids combines the ease of conventional cell models and the reliability of xenotransplants in vivo. In addition, it is less resources-consuming and more replicable with respect to xenotransplants.
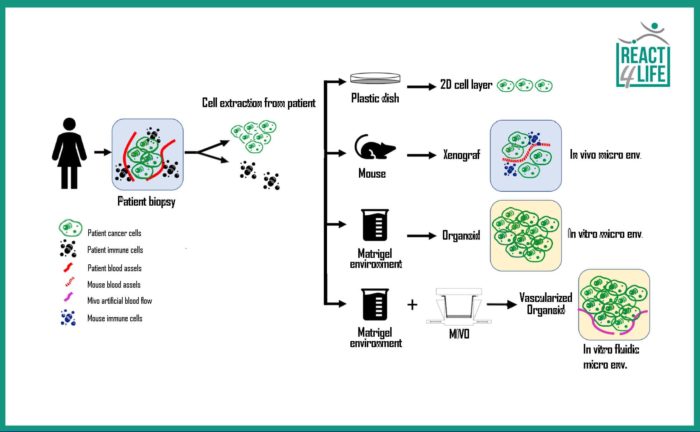
the options of modeling the cancer are: 2D culture on plastic, in vivo model via animals, and a 3D organoid:
the MIVO allows the artificial vascularization of the organoid making this model more realistic and reliable.
However, this model also has limitations: the main one is the lack of a real physiological microenvironment, consisting of gradients of growth factors and extracellular signals, immune cells, blood vessels for the tumor vascularization, inflammatory responses, etc.
In cancer research particularly, the microenvironment plays an important and essential role: micro-environmental factors can alter the survival of cancer cells and the drug response. To date, no solid and effective standard protocol for recreating an in vitro microenvironment for more reliable studies with organoids exists.
Certainly, there are intelligent solutions that can help scientists to simulate the physiological microenvironment, such as the co-culture of cancer cells with immune ones, the use of the correct concentration gradients of growth factors, and the implementation of a fluid-dynamic system that replicates the mechanical stimuli which the cells are subjected to in the in vivo condition.
From this point of view, React4life offers the ideal solution: the MIVO device allows the culture or co-culture of organoids in a fluid-dynamic environment capable of recreating, in vitro, the biochemical and mechanical stimuli due to the blood flow that pervades the tissues.
The combination of MIVO and organoids represents an innovative system suited to provide excellent results in the field of biomedical research. In addition, using MIVO device, researchers can test the effectiveness of different therapies by recreating the systemic administration of the drug that circulates in the blood flow before reaching the target tissue: this approach could open significant scenarios towards personalized medicine.

