The development of new research methods for drug analysis represents one of the most significant aspects of drug development. However, this complicated process still faces some challenges from significant issues such as time consumption, low-throughput, poor reliability and standardization, high cost.
The traditional 2D static culture in most cases do not predict the in vivo response as they cannot resemble properly the tissue physiological environment.
Therefore, the need of combining the fluid physics along with three-dimensional cell compartmentalization has led to the development of novel in vitro system such as lab on chip and microphysiological systems (MPS) like MIVO®. The basic idea is to provide a fluid-dynamic cell culture environment better resembling the human body, to boost drug discovery and disease knowledge. Moreover, the enormous potential to accommodate every type of cell into a physiologically relevant environment also fills in the gap between widely studied animal models and human-based clinical trials.
Lab-on-a-chips are currently adopted for the high-throughput screening of drugs. A plethora of microfluidic-based systems has been developed in the last years with the aim to facilitate and accelerate the in vitro assays and the early stage drug discovery.
The volume of a typical microfluidic chip device is minimal, and many functions can be integrated on a chip of few centimeters. The internal dimensions of average chip usually range from micrometers to millimeters, so the consumption of the samples and reagents is at the nanoliter and picoliter levels, making possible the study of a single cell to a few hundred cells, when macroscopic 2D cultures support 104 to 107 cells on a flat surface.
However, the low amount of cells and media volume adopted makes difficulòt their adoption in the late drug development and preclinical phase, where more complex and in vivo like environment are required to better investigarte the drug toxicity and efficacy MIVO® technology has been properly designed and developed to overcome some of these issues, by providing a valid platform for drug development studies.
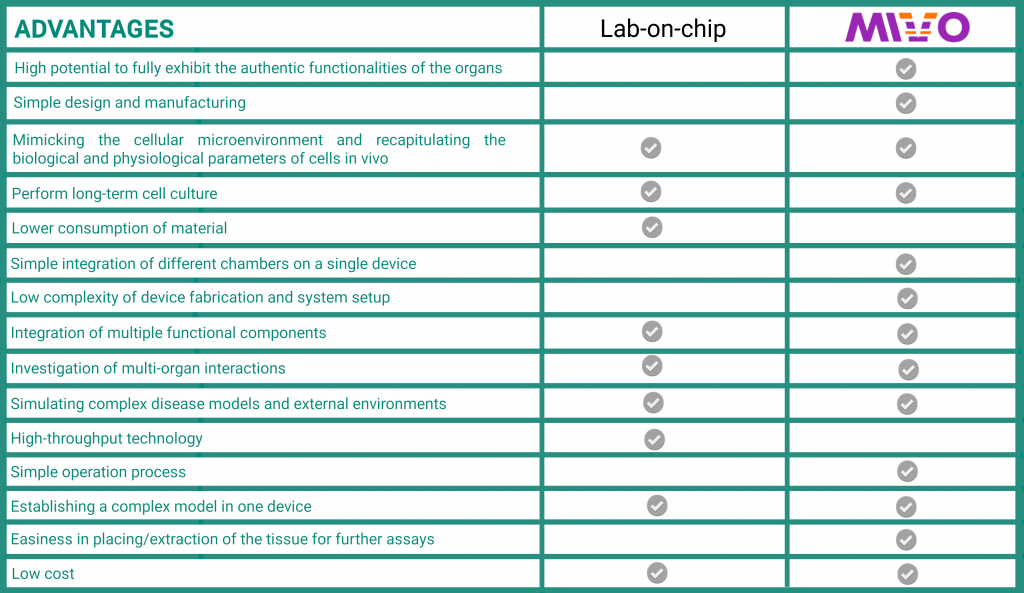
The MIVO® device is able to host 3D clinically relevant size tissue models (e.g. patient derived biopsies, bioprinted scaffolds, commercialized reconstructed tissues) and to provide the mechanical stimuli typical of the fluid-dynamic in vivo environment that are mainly due to the blood flow through the capillaries feeding the tissues and transporting nutrients and/or drugs. With this technology a larger amount of fluids is manipulated (up to few ml) which defines a flow rate ranging from ml/min to ml/min that make this device suitable for micro- and macro scale applications. As summarized in the table below, the advantages of MIVO® over microfluidic chips relate in particular to their simpler usability in terms of implementing the experimental setup and handling the cellular material. Moreover, in certain applications, due to the small size, the organs-on-chip are difficult to fully exhibit the authentic functionalities of the organs and not all the tissue/organ model commercially available can be integrated within these devices.
Using multiple MIVO® devices fluidically connected is also possible to reproduce a multi-organ configuration, where different type of cultured tissue shares the same simulated blood flow, for studying how organs interact in different experimental conditions.